 |
| https://upload.wikimedia.org/wikipedia/commons/thumb/8/85/Smiley.svg/2000px-Smiley.svg.png |
Sunday, February 28, 2016
Post Electronic Structure Quiz
Going into the quiz I felt pretty good. I did all of the practice quizzes and felt comfortable about the real quiz that was awaiting us on Friday. Going into it knowing it was 35 questions felt good. It allows a little room for error if there are a couple tricky ones. The actual quiz was somewhat tricky. The questions seemed to be very easy, except there was a lot of detail that you needed to pay attention too because it could've easily tricked you. All in all, I thought the quiz was okay. Hopefully the score reflects my thoughts.
Wednesday, February 24, 2016
Lessons on Electronic Structure
The first couple of lessons of this unit aren't as bad as a lot of other units we have had. The first lesson was primarily math. All we had to do was plug and chug with a couple equations that have to do with wavelength and frequency. Then we moved on to a little more complex stuff that involves electron orbitals. So far so good though, hopefully this unit will help me get back into the rhythm of chemistry.
Here is the wavelength equation:
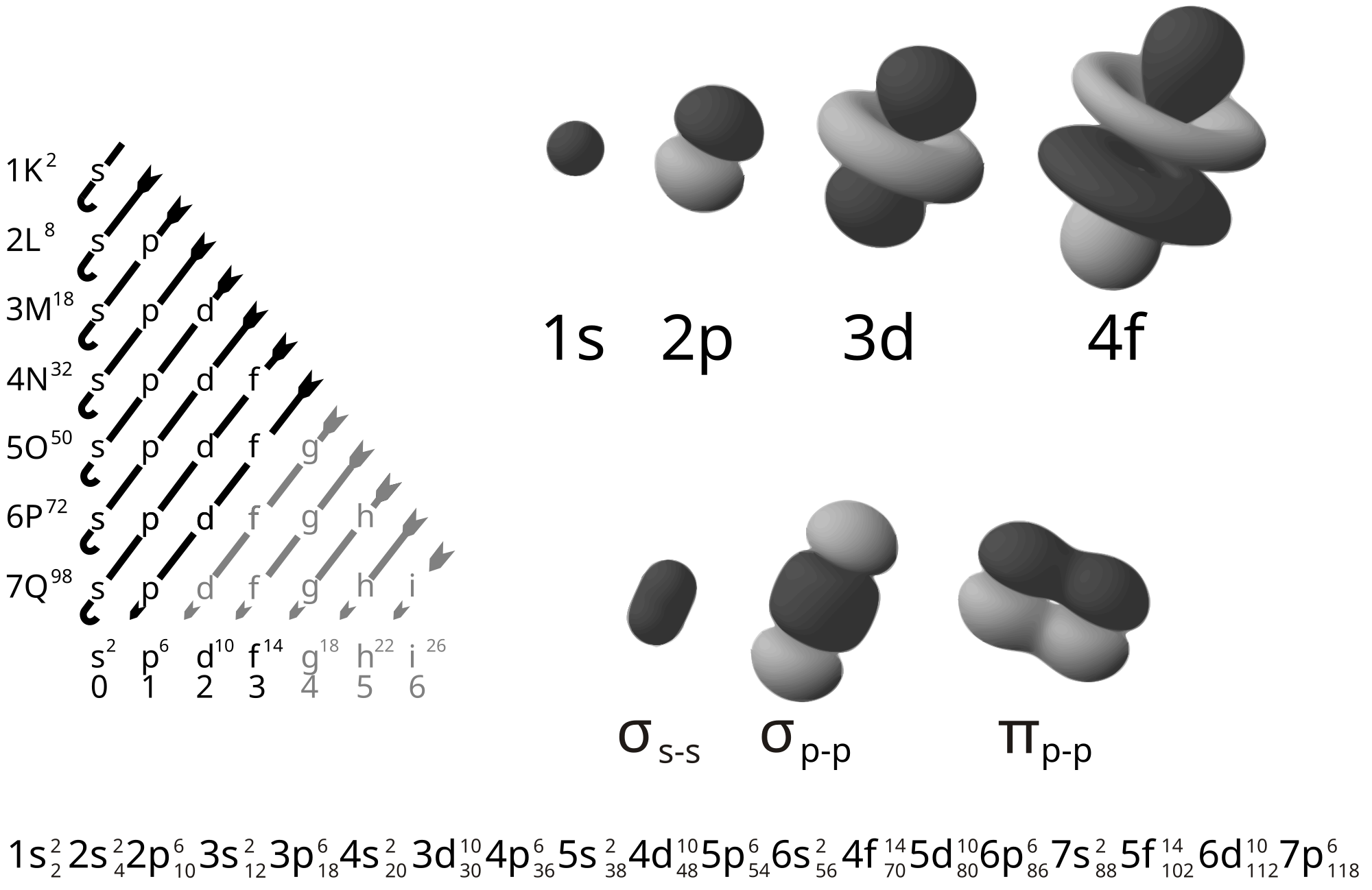
Here is electron orbitals:
Here is the wavelength equation:
| https://encrypted-tbn2.gstatic.com/images?q=tbn:ANd9GcT3DRXYikGd8jhZJFRVmum0AIs2awppkHZsfjWdeHwBkfRiEoG_ZQ |
 |
| https://upload.wikimedia.org/wikipedia/commons/thumb/1/11/Electron_orbitals.svg/2000px-Electron_orbitals.svg.png |
Monday, February 15, 2016
Overall Summary of Acids and Bases
Overall, it was a difficult, yet interesting unit. I personally was gone two days for field trips and those days were on the lab days. This was a good and bad thing. Good because I didn't miss any lessons, and bad because there was 3 questions from the lab we performed. Oh wells... Everything that was said about acids and bases was somewhat true. It was a difficult unit but I gave it my best effort. It was a good run Acids and Bases.....
| http://rs269.pbsrc.com/albums/jj77/carasian/bye-1.gif~c200 |
Sincerely,
Matt
Post Acid Bases Test
After I put my pencil down and turned it in, I was a little frazzled. All the stories told about the "worst chapter in the book" were true. It was a difficult chapter, not because the material was difficult to understand, but because of all the possible tiny mistakes you can make during the test/unit. The test for me could've gone either way.. Hopefully all is well and there is no sadness in the end of the day.
Wednesday, February 10, 2016
Test Study Links
A few links I used to study!!
Acids and Bases General Help
Titration
pH
Bronsted Acids and Bases
Arrhenius
Strong and Weak Acids and Bases
Acids and Bases General Help
Titration
pH
Bronsted Acids and Bases
Arrhenius
Strong and Weak Acids and Bases
Tuesday, February 9, 2016
Acids and Bases Titration Lab 2
The titration lab 2 is very similar to the last lab. Most people had to standardize the new solution but luckily we didn't have to because we already figured out the molarity in the last lab. This helped speed up the lab. Essentially we needed to find the molar mass of the unknown substance we had. We titrated the solution with the NaOH solution with the known molarity. We could then run the math and calculate the molar mass of the unknown substance. I thought this was a good experience because I missed the first day. This was complicated lab but I definitely needed it to help me get through this unit.
Monday, February 8, 2016
Acid Base Titration Lab 1
Last week on Wednesday through Friday we worked on a titration lab. It was one of the more complex labs we have done in the year and I missed it because I was on a field trip. So basically, we titrated KHP with NaOH. We used the mass of KHP and the volume of NaOH to calculate the molarity of the NaOH using the volume needed to titrate the solution. Since I was gone, I used another groups data which had 0.1930 as the Molarity of the solution. Part two of the lab consisted of us titrating acetic acid with the NaOH we had. Since we now know the molarity all we needed was to know was the volume of NaOH is needed to titrate the vinegar. The group's calculations came out to about 0.2318M for the Acetic Acid.
In the end, I do believe it was beneficial to do this lab because it would've helped me understand the unit more. Since I missed it, I am spending my time trying to learn what happened. Luckily we do another lab that is very similar Monday and Tuesday.
In the end, I do believe it was beneficial to do this lab because it would've helped me understand the unit more. Since I missed it, I am spending my time trying to learn what happened. Luckily we do another lab that is very similar Monday and Tuesday.
Thursday, February 4, 2016
Acid Base Quiz
So I've been convinced that the acid base unit is the most impossible thing in the world. Since I am somewhat scared I am trying to prepare myself a little more than I normally would. I prepared pretty well for the quiz so I was hoping for the best... I ended up with a B on the quiz so I was somewhat okay with it but I was hoping for better. Oh well, on to the test!
| http://s2.quickmeme.com/img/17/178221296212ba7cbeb70c9c020be3a52f59f3e93c370b78a06d545a224080c0.jpg |
Subscribe to:
Comments (Atom)


