Our new unit is Gas Laws. So far we have learned a few of the gas laws and I'd like to reflect on the first couple. The first is Boyle's Law, he discovered the relationship gases share between volume and pressure. The equation is simply P1V1=P2V2. It is a simple proportion that makes sense when you think about it. Here is a simple photo to explain the relationship.
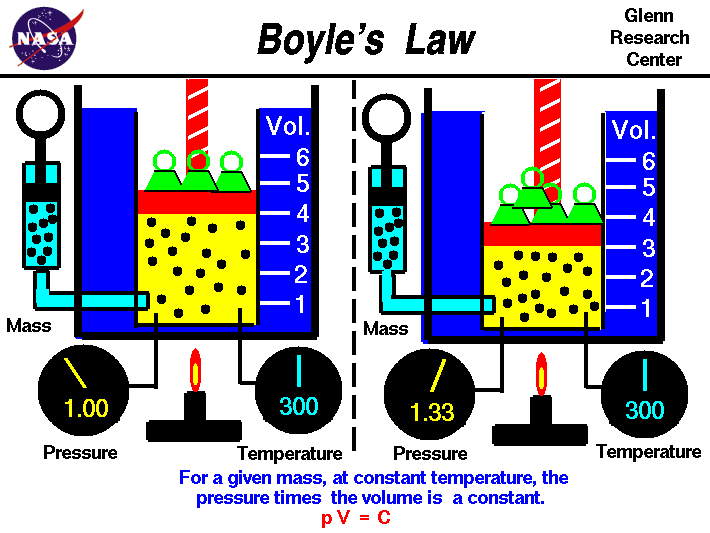 |
| https://www.grc.nasa.gov/www/k-12/airplane/Images/boyle.gif |
The next law we learned about is Charles' Law. Charles' Law shows the relationship that Temperature and Volume share: V1/T1=V2/T2. It is also simple to think about, When something is heated their particles get more excited and thus their volume is increased. Here is another photo that explains it simply.
 |
| https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEg6G_Jt0_9y3LtDq_T2xkH7pNjRkcB39o0TOOo6Ej_m-BugOlwq-TliuzG2862vuEhEXT1_AMyxQN1P8jMBZSsHQZqh_y6LEy50mLqS_oDJ3tBlDHvJdLquvXkCFR6LFjijKuCH3NL9exbl/s1600/CL+Double.png |


I agree this law is pretty easy to understand and think through. If I just think about what happens to volume when pressure increases, I understand it more than looking at the formula (but that's just me).
ReplyDelete